Search and Destroy: The Doom of Glioblastoma Multiform
Nic Zavala
In 2010, Glioblastoma Multiform (GBM) claimed 2,000 Americans and put more than 3,000 on its list. GBM is extremely aggressive and is a Stage IV malignant tumor. Patients diagnosed with GBM hardly make it past the first anniversary of diagnosis. A better understanding of GBM is urgent as many of these brain tumors are not discovered until they are in Stage IV and is taking over the brain (see above image).
GBM is mostly found as a primary tumor and is noted for its extreme motility and local invasiveness through the white matter of the brain. Healthy, adult neurons are normally terminally differentiated and have lost their ability to actively seek destinations as they do in early development. Not GBM cells who appear to have re-gained their functions of the past. Their motility renders a complete and successful resection of cancerous brain-matter extremely difficult that will end in novel tumor growth.
Invasion and the Culprits: Netrin, Laminin-1, and the DCC-receptor
[The article I will be referring to is "Autocrine Netrin Function Inhibits Glioma Cell Motility and Promotes Focal Adhesion Formation" and can be found at http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0025408 ]
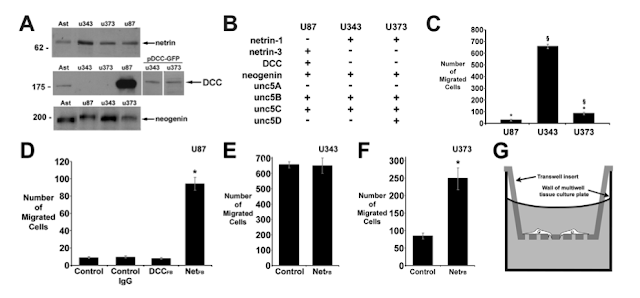
Netrin is a chemotropic axon guidance cue in early development. It tells emerging axons where to go, when, and where depending on varying gradients in vivo and in vitro. Later in healthy neuronal life, netrin may be implicated (according the attached study) in maintaining tissue integrity and structure as the researchers at McGill University found it was largely localized near Focal Adhesion sites (FAs) between cells or ECM. Netrin's receptors are DCC and UNC5.
1) McGill researchers obtained resected and immortal tumor cells from 3 different human cell lineages (patients, in this case). They used Western Blots (A) to confirm that each type of GBM cell was producing, and likely secreting, membrane bound netrins. They allowed cells to spontaneously migrate in this experiment without any added attractive/repellant factors. Only one of the lines, U87 (Stage III), was producing the DCC-receptor and far less capable of cell migration (C). U343 cells that lacked the DCC receptor migrated much more frequently (~7x more than DCC+ U87 cells). U343 are Stage IV cells...
** When Netrin was blocked by antibodies in the U87 culture, they were able to migrate more frequently (D). I thought the antibody usage as a blocker was creative but was disappointed by the swap of axis numbering. I realize they want to highlight a very interesting change, but a bit shameless if you ask me.
2) Here the McGill researchers demonstrate elegantly that netrin alone is a chemoattractant for cells. Focus on figure D. They did something really cool. The inserted an active DCC gene to U343 cells to use as an incredible control.
**You can see that generally any presence of netrin improved migration in GBM cells, if you had a DCC receptor. What I cannot wrap my head around is why U343 without DCC and with Netrin (C) would migrate 80% more than when they blocked DCC function in DCC-expressing "twin"?? Why is not the absence of DCC enough to improve migration? Maybe it their constant switching of axis that make this really hard to compare...AND the DCC twin (D) had 100% of the migration as the DCC- parental line under the same conditions!!
 3) Laminin is is a major component of the basement membrane and extracellular matrix. McGill researchers found that GBM cells preferentially migrate towards laminin and actually begin to REPEL from netrin for U87 cells that express DCC natively. Researchers noted "Importantly, this finding provides strong evidence that laminin-1 does not influence the response to a gradient of netrin-1 by arresting cell motility. They run to low concentrations"
3) Laminin is is a major component of the basement membrane and extracellular matrix. McGill researchers found that GBM cells preferentially migrate towards laminin and actually begin to REPEL from netrin for U87 cells that express DCC natively. Researchers noted "Importantly, this finding provides strong evidence that laminin-1 does not influence the response to a gradient of netrin-1 by arresting cell motility. They run to low concentrations"** When encountering uniform lamin distribution and descending gradients of netrin, DCC cells migrated further and towards the lesser concentrations of netrin...perhaps closer to the basement membrane and capillary walls. DCC is required for repulsion from netrin. Does this mean that Stage III cells expressing DCC are approaching the membranes and are about to become Stage IV?
-----
It turns out that if a cell expresses DCC it will migrate towards laminin in the capillaries and basement membrane. If one does not express DCC is more likely to migrate regardless of what is around. This makes fighting glioblastoma multiform incredibly difficult.
So who is the ultimate culprit? This study would suggest a loss-of-funtion in netrin... which could lead to disruption of tissue structure, cell-to-cell adhesion. But also that the loss of DCC (or having for that matter) is dangerous. I am a bit confused and am trying to look it up. I want to learn more about how this new found motility arises. I wonder if it is a gain of a novel function of DCC... netrin and laminin are always around in healthy cells but they take on new functions in GBM. So what is it? One thing is certain, this is not a cancer that you want. Detection is often too late and resection is one of the few treatment options because passing the blood-brain barrier with chemotherapy is not an easy task (Check out Jackie's post on Nanoparticles!!). But because GBM is so motile, some is often left over post-surgery and brings about a new tumor.
****WARNING, for those interested. I included a video I found of an open cranial surgery where they are removing (or attempting to) a GBM tumor. Its very real and a bit "graphic." Hence, the warning.