So it took me a while to find a cure to avoid aging, but with a lot of perseverance and the fact that anything can be found through Google, I managed to stumble upon an anti-aging drug. This drug, named AT-65, is a telomerase activator that rejuvenates your cells (T.A. Science). Unlike wrinkle cream, this drug takes the rejuvenating process to the extent that your very genetic make up is being effected. People who have been "treated" with this drug argue that they have regained eye sight, strengthened their immune system, improved sexual performance, increased brain speed, and maximized bone density. The science behind this drug originated from the study of telomerase, which became subject of great focus after Carol W. Greider and Elizabeth Blackburn were awarded the 2009 Nobel Prize in Physiology or Medicine for their discovery of telomerase (Callaway).
Whether this drug is a hoax or is an actual treatment to prevent aging is a matter of interpretation, but I hope skepticism will not get the better of you as you question the validity of this drug. My objective is to introduce you to this great study that illustrates why telomeres are involved in the notion of age prevention in order to formulate a conclusion of what telomerase enzymes do and if they can be manipulated into a tool that can be fashioned as the AT-65 anti-aging drug.
Background to understand the study:
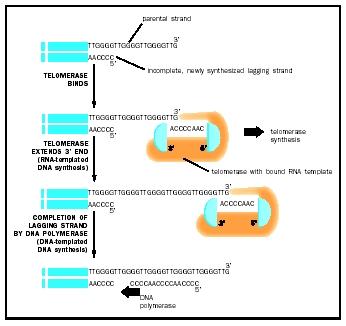
Telomerase are enzymes that cap the end of the chromosomes by adding sequence repeats to the 5’end of DNA strands; these caps are known as telomeres. Telomeres contain non-coding DNA that prevents the loss of valuable DNA from the chromosomal ends. The reason why the ends of the chromosomes are in constant danger of being damaged is due to the fact that each time the DNA is replicated, replication of the lagging strand is not completed. To prevent damage to the DNA, telomerase restores the original length of the chromosome by synthesizing DNA from a RNA primer to ensure that DNA is protected by telomeres.The information from this background section was obtained from the "The Biology of Cancer", Weinberg (pg. 368).
Here is the gist of this research (read the article though…it’s worth it! .)
The purpose of the study is to determine whether established multi-system degeneration in adult mice with telomerase dysfunction can be stopped or possibly reversed by re-activation of telomerase activity. The way this study was approached was by engineering a knock-in allele encoding 4-OHT inducible telomerase transcriptional receptors under control of the TERT promoter. The 4-OHT was found in the study to be necessary for sustaining active telomerase, and with this discovery the protocol of the experiment was set up. Two sets of mice were used in this research: G0 mice with an active telomerase that served as the control experiment, and G4 mice whose telomerase is defective. G0 or G4/vehicle in this study represents cells that have no 4-OHT administered, while G0 or G4/4-OHT do.
1) Assessing the impact of telomerase reactivation on telomere dysfunction-induced proliferative arrest
- G4 plated without 4-OHT
G4 cells adopted a flat senescent-like morphology. Proliferation of cells did not follow the cell cycle. Cultures accumulated mainly in the G0/G1 phase of the cell cycle.
- When replating these same cells in 4-OHT
This G4 cells experienced telomerase reactivation that induced telomere elongation. Proliferation records show reduction of in the G1/G0 phase fraction. Lowering of cycling-dependent kinase inhibitor, p21, allowed the re-entry of cells into the cell cycle, reduced apopotosis of tested germ cells and intestinal crypt cells, and reduction of tissue atrophy with restoration of testes and spleen size was experienced with an increase in fecundity.
- G0 were plated in the same conditions as G4
Treatment had no effect of these cells when 4-OHT was present.
Conclusion: This experiment reveals telomerase reactivation to be able to mark extinction of DNA damage signaling, improvement of cellular checkpoints along the cell cycle, and reversal of tissue atrophy in highly proliferative organ systems.
2) In addition to proliferative organs, brain health was studied since it’s the first determinant of age-progressive decline in humans. Accumulated DNA damage and progressive restriction of neurogenesis and impaired re-myelination is cause by the decrease in neural stem and cell proliferation and differentiation. NSCs from adult mice were the subject of study.
- G4 without 4-OHT
In comparison to G0 (control), G4 NSCs cells showed a decrease in self-renewal activity, smaller diameter, reduction of proliferating cells in the SVZ.
- When G4 treated with 4-OHT
Demonstrated restoration SVZ proliferation and telomeres elongation of SVZ, preservation of neural stem/progenitor reserves and an increase in neurogenic capacity.
Conclusion: Reactivation of telomerase activity can improve the limitations of neuronal proliferation and neurogenesesis.
3) Telomerase activation leads to tissue rejuvenation. The white matter of the corpus callus was the subject of study.
- Aged G4 had fewer Olig 2+ mature oligodendrocytes, which is associated with brain weight reduction. In addition, it was seen in G4 cells a significant thinning of the myelin sheathing of neurons.
- When G4 treated with 4-OHT
Reactivation of telomerase increased the number of oligodendrocytes to normal levels, alleviated the thin myelin level to mean standards and increased the size of brain.
Conclusion: telomere reactivation impacts ligodendrocyte proliferation and differentiation, and promotes repopulation of white matter structures.
4) The physiological and behavioral effect on mice of telomere dysfunction and reactivation on olfactory functions
- G0 mice avoid toxic 2-methylbutyric acid at all concentrations.
- G4 mice showed attraction/neutral behavior at concentrations lower than 1.87x10-4.
- G40 when treated with 4-OHT
G4 demonstrated avoidance behavior toward all concentrations of the chemical when reactivation of the telomeres took place.
Conclusion: this finding underlines evidence that when telomeres reactivate, SVZ neurogenesis and oligodendrocytes maturation occur, which in turn will promote the repopulation of olfactory neurons through myelination.
5) Physiologycal telomere activation effect across diverse adult cell types and organ systems
G4 cells that were activated with 4-OHT suppress DNA damaged signaling, restore cellular check points in several high-turnover organ systems. Thus, across different organ systems we can see the same results that were found in previous experiments when telomeres are activated.
From this study, in my opinion, the foundation of this drug can be seen to the point that one might wonder if its effects are in fact valid when looking at the data from this study. However, there are certain complications that I don’t quite understand when thinking about how this drugs function. One concern is that it is highly doubtful that telomeres are the sole structure that drives aging. Thus, using telomeres as an anti-aging tool should be coupled with other aging driving forces or at least the effect that telomerase manipulation has on other aging agents must be completely understood. Another issue that arises is the fact that telomerase activity is not as high in all cells of our body, rather it is more active in cells that experience rapid replication (Freeman, Biology of Science). Thus, the drug success might be limited, if it actually works, to areas of high proliferation. More importantly however, is the idea that delaying or preventing cell aging might have consequences that we might have not seen yet. It seems improbable that affecting the cycle of life in such a way could not have some serious implications. Nevertheless, it is necessary to say that with the discovery of telomerase and other revolutionary technology, the coming years might not only provide humanity with an anti-aging “cure”, but also innovative treatments that have the potential to treat age-related diseases.