 |
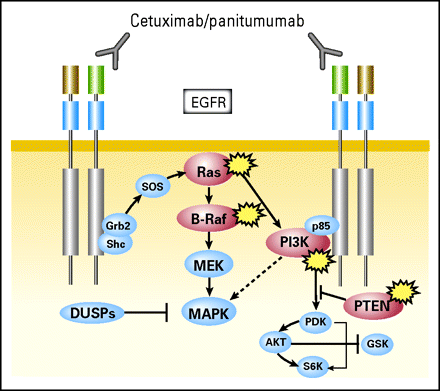
| Figure 1. Similarity in anti-EGFR treatments in disrupting MAP Kinase pathway. |
Furthermore, there was a phase 3 study conducted to determine if there was a difference between cetuximab and panitumumab on KRAS wild-type patients with metastatic colorectal cancer. As my partner and I noted in our wiki, KRAS mutations can have negative impact on anti-EGFR therapy, so all participating patients were wild-type KRAS. All 999 patients that were part of the final analysis had chemotherapy-refractory cancer. 499 patients received panitumumab and 500 received cetuximab. The primary analysis was overall survival, defined as the percentage of surviving patients from the start of treatment onward.
 |
| Figure 2. Kaplan-Meier plot of overall survival by treatment group. |
However, it is worth noting that panitumumab is given in 7 doses versus the 14 doses for cetuximab in the study.The minuscule difference in drug response may be attributed to the larger but less frequent doses of panitumumab having more efficacy. Regardless, the researchers concluded that the monoclonal antibody drugs are nearly identical in efficacy and safety (Price, et al. 2014). Yet the issue remains of how one drug's resistance does not occur simultaneously with the others (when the two are given together). If the monoclonal antibodies abide by the same mechanism, the discrepancy in resistance acquisition is possibly due to cetuximab and panitumumab binding to distinct epitopes. That is, the antibodies are binding to different antigen domains, leading to slightly different EGFR-binding capacities. Consequently, the EGFR-binding suppression occurs differently in the two drugs. Nevertheless this is only a hypothesis, and further work is being conducted to discover the difference in panitumumab (brand name Vectibix) and cetuximab (brand name Erbitux).
References
"Journal of Clinical Oncology." Molecular Mechanisms of Resistance to Cetuximab and Panitumumab in Colorectal Cancer. Journal of Clinical Oncology, n.d. Web. 07 June 2014. http://jco.ascopubs.org/content/28/7/1254/T1.expansion.html
"About FDA." Cetuximab (Erbitux) and Panitumumab (Vectibix). FDA, n.d. Web. 07 June 2014. http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm172905.htm
"Panitumumab versus Cetuximab in Patients with Chemotherapy-refractory Wild-type KRAS Exon 2 Metastatic Colorectal Cancer (ASPECCT): A Randomised, Multicentre, Open-label, Non-inferiority Phase 3 Study."Panitumumab versus Cetuximab in Patients with Chemotherapy-refractory Wild-type KRAS Exon 2 Metastatic Colorectal Cancer (ASPECCT): A Randomised, Multicentre, Open-label, Non-inferiority Phase 3 Study : The Lancet Oncology. The Lancet Oncology, n.d. Web. 07 June 2014. http://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(14)70118-4/fulltext
