The authors of the study were conducting the trail in 16 countries and 114 centers. Having large groups of patients is necessary for a phase 3 trial, whose main functions are to assess the drugs effectiveness, side effects, compare it to commonly used treatments, and gain information that will allow the drug to be used safely. The funding was completely done by Bayer, in hopes of getting the drug on the market.
Overall, 760 people over the age of 18 were randomized in the trial, with 505 getting regorafenib treatment (5 of which actually did not) and 255 getting placebo (2 of which did not). The patients chosen must have had cancers that progressed over 3 months while not receiving any form of therapy. The trial was also double blind; the randomization and statistics were kept hidden from the patients and investigators (and the sponsor) throughout the trial. The trial profile further illustrates the selection, randomization, and analysis of the patients.
All patients received as equal supportive care as possible while retaining efficacy. Additionally, each patient was given 160 milligrams of Regorafenib or matching placebo orally once a day over 3 weeks (of a 4 week cycle). After every 2 weeks the patients were assessed to determine if they should continue treatment. While no crossing over of treatment occurred, some patients did require a reduction in drug dosage due to treatment related toxic effects. Nevertheless the majority of baseline characteristics were the same in the Regorafenib and placebo groups, including the interventions given to the patients previously before the trial. In fact, all patients in the study had received Bevacizumab (a anti-VEGF therapy) sometime after their initial diagnosis of colorectal cancer. Thus the idea of different responses in patients because of different previous therapies can be ruled out.
The mean duration of treatment was 2.8 months for the group receiving Regorafenib and 1.8 months for the group taking placebo. After 432 deaths the primary analysis was obtained. The primary analysis was overall survival, defined as the time (in days) from the point of randomization to the time of death of any cause. The median overall survival for patients in the regorafenib group was 6.4 months, whereas the median survival for the placebo group was 5.0 months.
 |
| Figure 1: Overall Survival |
Over the trial period, it appears regorafenib was helpful in treating patients, but the long term survival rate shows the opposite. In fact, after a year of therapy, the placebo has a greater long term overall survival percentage then the group treated with regorafenib. However, it is important to keep in mind the number of individuals in the study is much lower by 12 months. Only 3 patients of the placebo group are in the trial after a year (only 7 in the Regorafenib group). One may infer that regorafenib hinders too much cell replication over time compared to a placebo. In which case the repeated cell death is causing the lack of long-term survival compared to the placebo. Another possibility is that more patients, especially late in the trial, died due to treatment-related side effects.
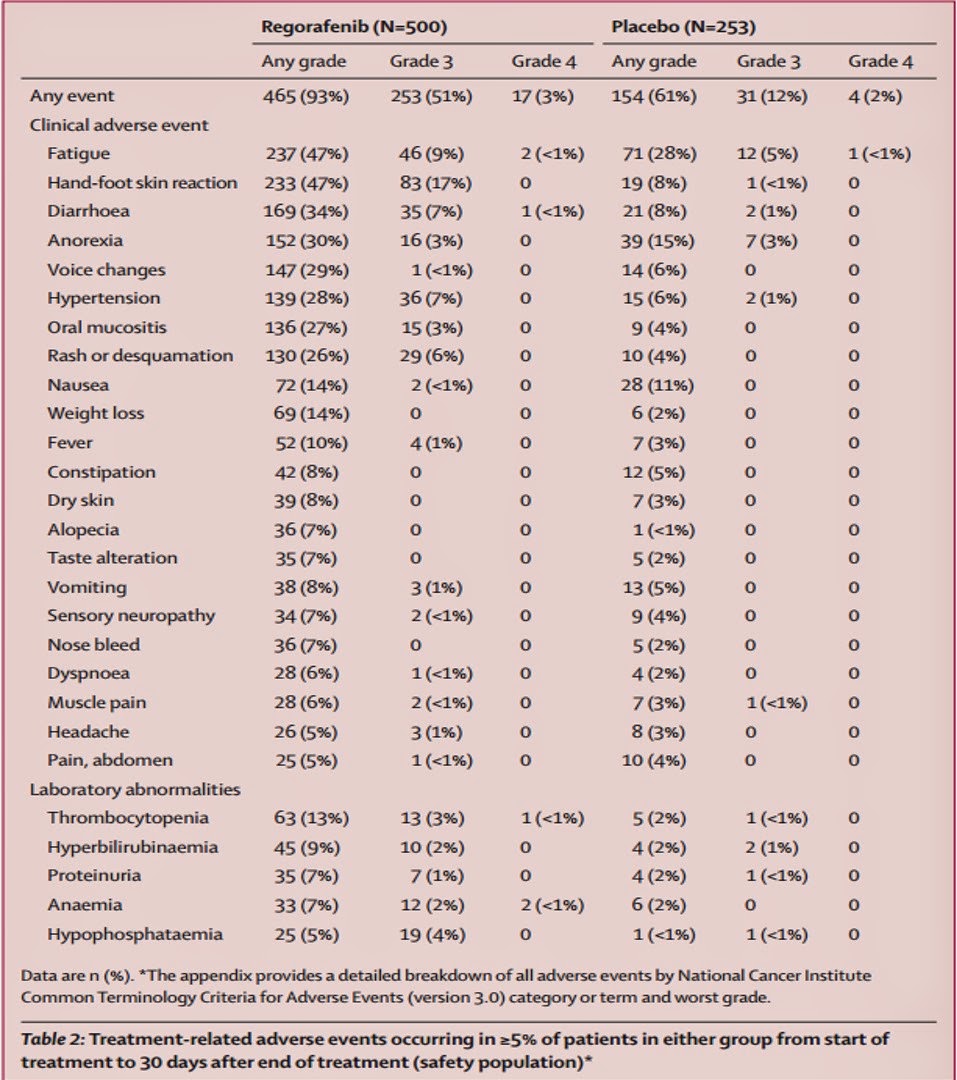
There were treatment-related adverse effects in 465 patients (93%) taking regorafenib and 154 (61%) in those taking placebo (Table 2). The most common adverse effect with regorafenib is hand-foot skin reaction and fatigue. Based on the high percentage of hand-foot skin reactions, it appears that regorafenib might inhibit cell surface kinases in the extremities (Table 2). In the placebo the most common side effects were fatigue and anorexia. However, most adverse effects occurred in the beginning of treatment. If the start of treatment is monitored carefully the drug can possibly be used more safely. Also, when a patient has colorectal cancer at such an advanced stage, their life will be difficult regardless. Therefore, having 6.4 months versus 5.0 months on average is a significant improvement for patients without any previous way of treating their late-stage cancer. Some patients will gladly take an extra month of life.
Following FDA regulation last year, Bayer developed regorafenib into their drug Stivarga. Researchers hope it will be the basis for a new standard of care for patients with late-stage metastatic colorectal cancer. There are still some challenges, such as identifying the exact mechanism of the reaction between regorafenib and tumors. And researchers need to understand why there were differences in the responses of some subgroups. With all of this research underway, regorafenib (Stivarga) and other multikinase inhibitors are leading the way for therapy in patients with treatment-refractory cancer.
References:
1. "The Lancet." Regorafenib Monotherapy for Previously Treated Metastatic Colorectal Cancer (CORRECT): An International, Multicentre, Randomised, Placebo-controlled, Phase 3 Trial : The Lancet. N.p., n.d. Web. 29 May 2014.
2. "Patients With Metastatic Colorectal Cancer Treated With Regorafenib or Placebo After Failure of Standard Therapy." Home. Bayer, n.d. Web. 29 May 2014.
3. "The Next Proven Step." STIVARGA® for Professionals. Bayer, n.d. Web. 29 May 2014.
4. "FDA Approval for Regorafenib." National Cancer Institute. NCI, n.d. Web. 29 May 2014.